CDSCO Import License Consultant for Medical Devices
CDSCO Import license for medical devices are issued under the Directorate General of Health Services in the Ministry of Health and Family Welfare.
If you want to accelerate your CDSCO Import licensing process then you can contact the Indiaxis team. Indiaxis team is a leading CDSCO Import license consultant in India. The team of Indiaxis company will assist you at every stage to get a medical device import license from CDSCO.
Free CDSCO Registration Consultation by Expert
Overview of CDSCO Import License for Medical Devices In India
Whenever you import medical devices in India you must follow all compliance rules for medical devices which are set forth by the CDSCO. The CDSCO department is only responsible for the approval and regulation of all medical devices and clinical trials in the country, laying down the standards for Drugs, and medical devices controlling the quality of imported medical devices, and coordinating of the activity of State Drug Control Organizations.
According to the new guidelines of the CDSCO Department, suspension and cancellation of CDSCO import license for medical devices for classes A, B, C, and D. It’s may happen if the importer of medical devices fails to fulfill the deadlines which are specified by CDSCO for a mandatory import license.
The CDSCO Department has already set a complete process for granting CDSCO import licenses for medical devices in India. This process applies to all types of medical device importers/distributors who import medical devices from other countries to India. However, all medical devices need to be classified according to the CDSCO classification rule. Some time ago medical device importers were able to sell out their devices in India without following any mandatory rules and regulations. But from 2006 onwards medical devices were banned from entering India if you do not follow specific import license guidelines which is set by CDSCO.
In India, there are several companies who’s import raw materials, semi-finished products, or other types of components. To market these as a finished medical device, getting a CDSCO manufacturing license is mandatory for the final assembly and packaging processes in India.
CDSCO Import License Registration for Medical Devices
- Pre-requisites for registration process:
- Generic Name / Brand Name
- Intended Use
- Material of construction
- Mode of application
- Study of device details and Classification of medical devices on the basis of risk of product.
Latest CDSCO Update
In collaboration with TCS and Invest India, India’s Directorate General of Health Services has started the National Single Windows System (NSWS) Portal, to simplify and streamline the CDSCO import license for media devices approval process. This process is effective from 1st January 2024, stakeholders are advised to transition to this user-friendly portal, available at https://www.nsws.gov.in, as the existing CDSCO md online platform will be deactivated by January 15, 2024.
Ordinance for CDSCO Import License for Medical Devices in India
CDSCO Import License for medical devices is an ordinance in India that any industry or individual who has the license (wholesale Form 20/21 B and/or registration certificate to sell any medical device in India form 41/ 42 can apply for a CDSCO import license under Central Drugs and cosmetics act, 1940 and can import any medical devices into India.
To register medical devices in India Foreign manufacturers have to appoint importers or authorized agents in India. In amendments clearly specified which documents are required for CDSCO Import License registration. The validity of the CDSCO Import License is 5 years from the date of the license issued.
Phases of CDSCO Import License Registration for Medical Devices
According to the CDSCO Regulation, registration of medical devices in India requires a certain mandatory requirement for regulatory certificates from foreign producers.
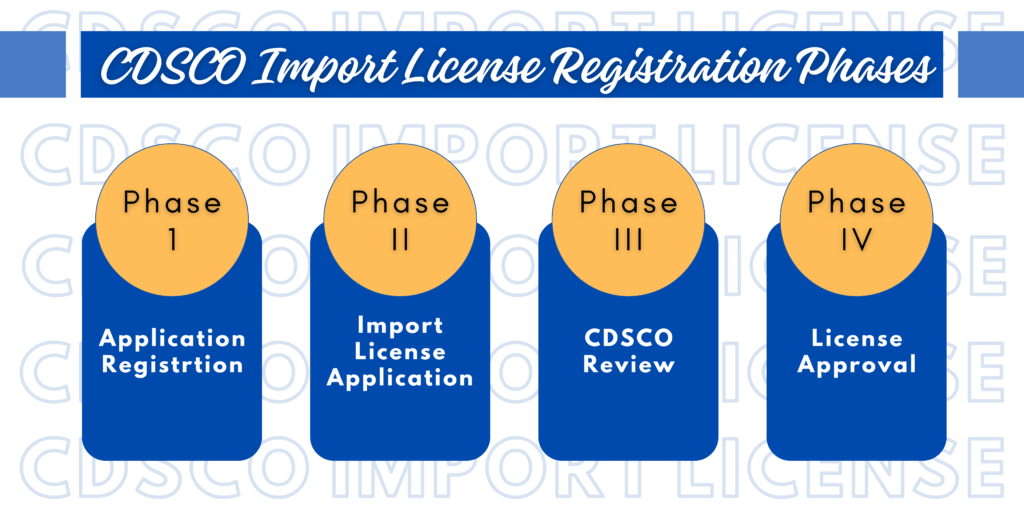
Phase I – Applicant Registration:
The client will have an active account in the CDSCO online registration portal for the registration of the applicant to the CDSCO portal. An authorized agent/distributor (who must have wholesale drug license form 20/21B or registration certificate form 41/42 to sell medical devices in India) shall make an application for the grant of a CDSCO import license for medical devices to the Central Licensing Authority through an identified online portal of the Ministry of Health and Family Welfare in the Central Government in Form MD-14 for obtaining a CDSCO Import license.
The procedure of Phase 1
- Online form submission.
- Submission of the required documents.
- Approval from CDSCO.
- If the application is rejected, CDSCO shall communicate its reasons for refusal or reapplication where necessary.
Phase II – Medical Device Import License Application:
Permission to import medical devices for commercial purposes shall be required when applying for an import license.
The procedure of Phase 1
- Submission of the online form with necessary details like classification of the device, brand name, intended use, product description, etc.
- Upload the documents, such as a certificate of European CE, free sale certificate, ISO 13485 plant master file, device master file etc. in accordance with Indian CDSCO’s regulatory norms.
- The CDSCO fees are to be paid on the basis of the product classification.
- After all documents and a copy of the fee invoice have been submitted successfully, an application number will be generated.
Phase III – Review from CDSCO:
CDSCO will review the application along with verification of all documents which are uploaded and if any query is observed they will communicate.
Submitting relevant justification or revised documents to CDSCO online portal.
Phase IV– License Approval:
CDSCO will review the justification along with verification of all documents and if it is as per CDSCO rule they will grant license.
What will Indiaxis be able to do for you in registering a CDSCO import licence of the medicinal device?
Indiaxis is a major regulatory consultant for the healthcare industry. In India, Indiaxis assists manufacturers in obtaining the necessary permits to import and sell medicinal products. Licences in respect of medical devices include 14 licences, 26 licences, 42 licences, and import licences. Indiaxis will assist clients in correctly classifying medical devices, setting up a CDSCO login credential, preparing all technical documents, reviewing and uploading any technical documentation to successfully apply for online payment of the CDSCO fees. Over the past 12 years, Indiaxis has been working closely with CDSCO and to date successfully registered 200 plus CDsCOs. In case of a query from the CDSCO, the Indiaxis Technical Team has inhouse ability to resolve it.
Competencies at Indiaxis
- To save customers’ commercial components and efforts, Indiaxis offers end-to-end guidance and assistance.
- Additionally, Indiaxis boasts a committed and knowledgeable regulatory staff.
- Indiaxis represents customers in USFDA, ISO 13485, and European CE audits.
- Indiaxis strategically strives for timely project completion and high-quality service.
Receive professional help, complete tasks without delay, and follow the right process to achieve desired outcomes.
Turnkey services, system implementation, licencing, training, and regulatory clearances and certifications such as FDA 510(k), European CE Marking, ISO 13485, SFDA, Egypt registration, etc. are all offered by Indiaix. Indiaix has a worldwide presence in 32 nations that import medical equipment from India, including Saudi Arabia, the USA, Oman, Qatar, Japan, the Philippines, China, and Egypt.
For further information on the CDSCO Import Licence, please contact us at +91 9013803615 via WhatsApp.
FAQ’s for CDSCO Import License Consultant for Medical Devices
How can I import my medical device to India?
To import medical devices into India, you need to register the manufacturing site and medical device with the CDSCO and obtain an MD-15 Import License. You can apply for the license by:
- Appointing an authorized Indian agent with a license to manufacture (for sale or distribution) or license to wholesale (sale or distribution)
- Filling out Form MD-14 on the Sugam online portal
- Providing documents based on class
- Paying fees
- Responding to queries
- Getting a review from the Medical Device Advisory Committee (MDAC) for novel products
The MD-15 license makes you the importer of the medical device in India.
As of October 1, 2023, all Class A measuring/sterile, B, C, and D medical devices require an MD-14/MD-15 Import License with the CDSCO before importing into India. Class A non-measuring/sterile do not require an Import License but do need to be registered through an online portal. The process for importing medical devices in India includes:
- Purchase Order or LC
- Certificate of Analysis
- Certificate of Origin
- Insurance Certificate
- Catalogue/Technical write up
- Tracking the Shipment
- Arrival Of Cargo
The National Single Window System (NSWS) Portal will simplify and streamline the approval process for the medical devices sector, effective from January 1, 2024. The existing cdscomdonline platform will be deactivated by January 15, 2024.
What is the license to import medical devices?
In India, the MD-15 license is required to import medical devices into the country. The license is issued by the Central Licensing Authority (CLA) to an Indian authorized agent or distributor. The MD-15 license is valid for five years after it is granted.
The application for the MD-15 license is made in an MD-14 form. The CLA will share any information that is insufficient with the applicant within 120 days, and will inform the applicant in writing within 30 days for reasons to be given in writing. The applicant will have 90 days to respond to the required information shared by the CLA.
The MD-15 license grants the license holder a comprehensive list of all approved medical devices for the manufacturing site. The license also allows the holder to import and sell medical devices and in-vitro medical devices in India.
A fee must be paid for the Single Device Registration in India. An additional fee must be paid for each additional device. The fee is paid via a challan as prescribed under the said rules.
How do I get medical devices approved by CDSCO?
The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body in India that approves medical device manufacturing licenses. To get a medical device approved by the CDSCO, you can follow these steps:
- Register as an Indian agent on the SUGAM online portal
- Pay the prescribed fees online for the manufacturing site and the proposed devices
- Submit an online application in Form MD-14, along with the required information
- Submit technical documentation for review by the CDSCO
- Register all of your device manufacturing facilities
- Submit hard copies of ID proof, undertaking, and address proof document to the CDSCO office
- The CDSCO will review the application and may raise queries if it is not satisfied
- The CDSCO will provide an IPC test letter
- Submit samples to IPC labs and once the IPC report is received, submit the application via form CT-21 to receive approval in CT-22 (API)/CT-23 (Formulation)
The CDSCO ensures that all medical device manufacturers and importers meet the requirements for their devices before they reach the consumer.
What is a medical device license?
A medical device license is a license issued by the Central Drugs Standard Control Organization (CDSCO) that allows a manufacturer to import or manufacture small quantities of medical devices for clinical investigations, tests, demonstrations, evaluations, or training. The license also allows the manufacturer to gather data on the device’s safety and efficacy.
To manufacture a medical device, a person must apply for a Test license by filling out a Form MD-12 from an online portal of the Ministry of Health and Family Welfare. The Ministry of Health and Family Welfare will then issue a Test license in the Form MD-13.
The CDSCO is responsible for the registration and regulation of medical devices in India under the Drugs and Cosmetics Act of 1940. Some of the CDSCO’s functionalities include:
- Granting a Certificate of Registration for a Notified Body
- Granting an Import License
- Granting a License or Loan License to Manufacture for Sale or Distribution
As of April 1, 2020, all medical devices must be registered under The Drugs and Cosmetics Act of 1940. Starting October 1, 2022, all Class A and B medical devices must have Import Licenses prior to importation. All remaining Class C and D devices not already Notified will need to have Import Licenses by October 1, 2023.
What is the duration of import Licence?
In India, an import license is typically valid for 24 months for capital goods and 18 months for consumables, spare parts, and raw materials components. The licensing authority issues an import license in Form 10 or Form 10-A, and it is valid for three years from the date of issue, unless it is sooner canceled or suspended.
You can apply for an import license if you are:
- A manufacturer with a valid wholesale license of drugs
- The manufacturer’s agent in India with a valid license under the Drugs and Cosmetics Rules, 1945
- The manufacturer’s agent in India with a valid wholesale license for sale or distribution of drugs
The licensing authority will issue an Import License in Form 10 within three months of receiving your application.
How do I bring my medical device to market?
To bring a medical device to market, you need to: Conduct market research, Develop a product marketing strategy, Obtain regulatory approval, Develop a sales strategy, and Launch and monitor your product.
Here are some strategies for marketing your medical device:
- Determine your company’s brand
- Create an online presence
- Expand your target customer beyond doctors
- Look beyond patient outcome
- Create a stream of quality content
- Compare your competitors’ strategies
- Weave story into statistics
What is the CDSCO fee for medical devices?
The Central Drug Standard Control Organization (CDSCO) defines the application fees for each category of medical device. The fees are:
- Class A or Class B: 5000 INR for one manufacturing site, and 500 INR for each device
- Class C or D: 50,000 INR for one site, and 1000 INR for each device
- Class A: US$1,000
- Class B: US$2,000
- Class C and D: US$3,000
The CDSCO also charges a professional fee of 99,999 INR for a CDSCO import certificate for cosmetic products. You can register in India through a local authorized representative or importer with the necessary licenses for medical products. You can also set up your own office to register directly. This is for informational purposes only. For legal advice, consult a professional.
List of CDSCO medical device classifications
The CDSCO classifies medical devices into four classes based on risk level:
- Class A: Low risk
- Class B: Low moderate risk
- Class C: Moderate high risk
- Class D: High risk
Examples of Class A devices include absorbent cotton wool. Examples of Class B devices include digital thermometers, needles, and suction cannula. Examples of Class C devices include bone plates and bone screws. Examples of Class D devices include heart valves.
How to apply for CDSCO certificate online?
To apply for a CDSCO certificate online, you can create an account on the SUGAM portal at https://cdscoonline.gov.in/. Then, you can:
- Fill out the application form with details about your product, manufacturing processes, and other relevant information
- Submit ID proof, undertaking, and address proof documents in hard copy to the CDSCO office
- Check your registered email for verification
- CDSCO will approve your registration after evaluating the submitted documents
You can attach the following documents to your registration application:
- ID proof document
- Undertaking issued by a government authority
- Address proof document
- Copy of BA/BE site registration as approved by CDSCO
- Manufacturing license or wholesale licenses in case of import or manufacture of drugs/blood product registration/test license registration
- Covering letter
- Description of the product
- Test protocol
- Quality certificates
- Instructions for use
- Fee challan
- Legal form
- ISO-13485
- PMS surveillance
A CDSCO license is valid for five years from the date it is granted or renewed.
How do I get CDSCO medical device classification list?
The Central Drug and Standards Authority of India (CDSCO) classifies medical devices based on risk. The categories are:
- Class A: Low risk, with minimal or no invasiveness, such as thermometers
- Class B: Low to moderate risk, with minimal invasiveness, such as needles and suction cannula
- Class C: Moderate risk, such as bone plates and bone screws
- Class D: High risk, such as heart valves
The CDSCO also classifies medical devices by category, including:Anesthesiology, Pain management, Cardiovascular, Dental, Ear, nose, and throat (ENT), Gastroenterological, Urological, General hospital, Operation theater (OT), and Respiratory. The FDA classifies medical devices into three classes based on risk:
- Class I: Low risk, such as bandages, handheld surgical instruments, and nonelectric wheelchairs
- Class II: Intermediate risk
- Class III: High risk, such as devices that are important to health or sustaining life