In the landscape that sometimes develops medicines, medical equipment, cosmetics, and health products, compliance with national regulatory standards is not only important, but it is necessary. In India, the Central Drugs Standard Control Organization (CDSCO) Apex Regulatory Authority, is responsible for ensuring the safety, efficacy, and quality of medicines and medical equipment. For manufacturers, importers, and exporters to enter the Indian market, it can be difficult to navigate the complex CDSCO approval process. This is where a CDSCO consultant plays an important role.
What is CDSCO?
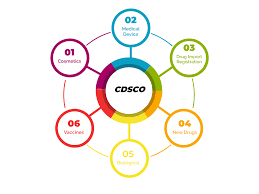
Central Drug’s Standard Control Organization (CDSCO) works under the Ministry of Health, the Indian government. It controls drugs, medical equipment, cosmetics, diagnostics, and organic products such as vaccines and blood products. The Drugs Controller General of India (DCGI) is the head of CDSCO.
- CDSCO is responsible for:
License for the production, import, and marketing of medicines and medical equipment.
- Regulation of clinical studies.
Ensure quality control through inspection and revision.
- Care of the Pharmacovigilance system.
Due to the technical and legal complications involved, most companies depend on a CDSCO consultant to handle approval and ensure complete compliance.
Who is the CDSCO advisor?
A CDSCO consultant is a regulatory specialist or company specializing in the end-to-end regulatory process required to approve and obtain CDSCO in India. These consultants work closely with companies to streamline the match journey for medicines, medical equipment, cosmetics, diagnosis, and more.
Whether it is registration, documentation, import licensing, test report, or website audit, a CDSCO consultant works as your match partner to simplify each step.
Services Offered by CDSCO Consultants
- A professional CDSCO offers a wide range of services that suit the needs of the advisory industry:
- Product classification and qualifying check
- Determine whether the product comes under the CDSCO regulator scope.
- Classification of medical equipment (Class A, B, C, D) for the correct licensing requirements.
- Regulatory documentation
- Preparation and review of doses, application, and technical documents.
- Ensuring that all submissions meet the CDSCO format and guidelines.
- Import/Production License
- Import License (Form 10, Form 41)
- Production License (Form 28, Form 25)
- Registration of medical equipment (MD-14, MD -15)
- Clinical test approval
- Help secure permits to conduct clinical studies.
- Ethics Committee’s approval and testing of surveillance support.
- Cosmetic product registration
- Guidance for cosmetic registration (Form 42).
- Component compliance, labeling requirements, and test support.
- Website Inspection and GMP -Society
- CDSCO Audit and preparation for inspection.
- Secure for good production practices (GMP).
- Market monitoring and pharmacovans
- Support in reporting side effects and PMS documentation.
- Management of the updating and renewal of the license.
Why Hire a CDSCO Consultant?
- CDSCO advisor is not a legal requirement, but it is highly recommended. Here’s the reason:
- Specialization in Indian regulations: Regulatory requirements in India are strict and often updated. An advisor is still up to date and avoids expensive errors.
- Speed and efficiency: CDSCO approval can take a week or a month. With the right documentation and strategy, a consultant can significantly reduce the deadline.
- Avoid rejection: Incorrect submission leads to delay or rejection. Advisors ensure proper filing and compliance to avoid errors.
- End-to-end support: From product classification to license delivery, advisors provide one-stop solutions.
- Cost-effective: Errors in the submission of regulations can lead to revocation or legal issues. Advice first helps save money in the long term, and corrects for the first time.
Who Needs a CDSCO Consultant?
- If you belong to one of the following categories, the hiring of the CDSCO consultant may be a game-changer:
- Pharmaceutical companies (domestic or foreign)
- Manufacture of medical equipment
- Cosmetic brand imported to India
- Start-up of health services, launching new products.
- Contract Research Organization (CRO)
- Hospitals and clinical laboratories working with regulated medical equipment
Choosing the Right CDSCO Consultant
While choosing a CDSCO consultant, see:
Proven experience with CDSCO license procedures.
Knowledge of international and Indian regulatory standards.
Timelines for transparent pricing and distribution of services.
The ability to represent your company during a revision or hear if necessary.
Good relationship with CDSCO officials.
Agencies such as Solutionplats, Cliniexperts, Operon Strategist, and others have created a reputation as reliable CDSCO consultation companies in India.
Conclusion
Compliance with CDSCO regulations is important for any company to enter or run the Indian pharmaceutical and health care system. A knowledgeable and experienced CDSCO advisor acts as your regulatory spine and helps you overcome complex laws, reduce the risk, and enter the market with confidence.
Whether you are a start-up or an international player, the right CDSCO partnerships with the consultant ensure that your products meet Indian standards and reach consumers efficiently and legally.