The CDSCO (Central Drugs Standard Control Organization) Manufacturing License is a vital aspect of ensuring that pharmaceutical products and medical devices manufactured in India meet the necessary quality standards and are safe for human use. This license is required for any manufacturer intending to produce drugs, medical devices, or other regulated products in India. It is an essential regulatory requirement for both domestic and international businesses operating in the Indian healthcare sector.
What is the CDSCO Manufacturing License?
The CDSCO Manufacturing License is an authorization granted by the Central Drugs Standard Control Organization, the national regulatory authority for pharmaceuticals and medical devices in India. This license is issued to manufacturers who meet specific criteria set by CDSCO and is required for the manufacturing of drugs, biologics, and medical devices. The primary purpose of this license is to ensure that the products manufactured are safe, effective, and comply with the Indian Drug and Cosmetic Act, 1940, and the rules framed under it.
CDSCO is responsible for regulating the manufacture, sale, import, and distribution of drugs in India. As a part of its regulatory functions, it ensures that products manufactured or imported into the country adhere to strict quality and safety standards. The manufacturing license also serves as a tool for the government to monitor and control the pharmaceutical industry, preventing substandard or counterfeit products from entering the market.
Types of CDSCO Manufacturing Licenses
- Drug Manufacturing License: This is the license required for the production of pharmaceutical drugs in various forms, including tablets, capsules, injectables, ointments, and syrups. It is further categorized into:
- Formulation License: For manufacturers who produce the final drug product (formulation).
- Active Pharmaceutical Ingredients (API) License: For manufacturers who produce raw ingredients or active substances used in drug formulation.
- Bulk Drugs License: For manufacturers producing larger quantities of drugs or raw materials for distribution to other manufacturers.
- Medical Device Manufacturing License: With the increasing demand for medical devices, manufacturers involved in the production of diagnostic devices, surgical tools, implants, and other medical equipment must obtain this specific license.
- Cosmetics Manufacturing License: For manufacturers producing cosmetic products in India, a specific manufacturing license is required under CDSCO to ensure that these products are safe and comply with the standards.
- Biologics Manufacturing License: This license is needed for manufacturers producing biological products such as vaccines, blood products, and other biologically derived substances.
The Importance of the CDSCO Manufacturing License
- Ensures Product Safety: The primary function of the CDSCO manufacturing license is to ensure the safety and efficacy of drugs, medical devices, and cosmetics produced in India. Only manufacturers who meet the stringent requirements of CDSCO are granted this license. This helps in preventing the circulation of substandard or unsafe products in the market, which could be harmful to the public.
- Quality Assurance: The license mandates that the manufacturing facility follows Good Manufacturing Practices (GMP) and other regulatory standards. This ensures that the products produced are of high quality and meet the specifications laid down by regulatory authorities.
- Regulatory Compliance: Obtaining a CDSCO manufacturing license ensures that the manufacturer complies with the Indian Drugs and Cosmetics Act, 1940, and other associated regulations. This helps the manufacturer stay in line with national laws and avoid legal liabilities.
- Market Access: For businesses wishing to distribute their products in India, obtaining the CDSCO manufacturing license is a prerequisite. It allows manufacturers to access a wide domestic market and, in many cases, international markets as well.
- Credibility: A CDSCO manufacturing license is a mark of credibility and trustworthiness. It assures customers, healthcare providers, and regulatory bodies that the manufacturer follows the necessary regulations and standards in their operations. Having the license can significantly improve the reputation of a manufacturer in the industry.
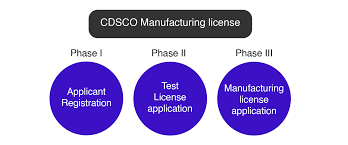
Steps to Obtain a CDSCO Manufacturing License
Obtaining a CDSCO manufacturing license involves several steps and documentation requirements. Below is a general outline of the procedure:
- Application Submission: The first step in obtaining a CDSCO manufacturing license is to submit an application to the CDSCO office. The application must include detailed information about the manufacturing unit, the type of products to be manufactured, and the facilities in place.
- Submission of Documents: Along with the application form, the manufacturer must submit several documents such as:
- A copy of the company’s incorporation certificate.
- Layout of the manufacturing premises.
- A list of key personnel in charge of the manufacturing process.
- A declaration that the manufacturing facility adheres to GMP standards.
- Evidence of compliance with environmental and safety regulations.
- Details of testing and quality control measures.
- Any other specific documents as per the CDSCO’s requirements.
- Inspection of the Manufacturing Facility: CDSCO authorities conduct a thorough inspection of the manufacturing facility to verify that it meets the necessary standards. The inspection process includes evaluating:
- The cleanliness and hygiene of the premises.
- The adequacy of equipment and machinery.
- The storage and handling procedures for raw materials and finished products.
- The qualification of staff involved in the manufacturing process.
- Record-keeping practices.
- Adherence to GMP and other regulatory requirements.
- Approval from State Licensing Authority: Before the CDSCO issues the final manufacturing license, the application and documents must be reviewed and approved by the State Drug Control Authority. Once the state authority approves the license, it is forwarded to CDSCO for the final review and grant.
- Issuance of License: If the inspection is successful and the documentation is in order, CDSCO will issue the manufacturing license. The license will be valid for a specific period and needs to be renewed periodically.
Key Regulations and Compliance for CDSCO Manufacturing License
- Good Manufacturing Practices (GMP): The facility must comply with GMP guidelines to ensure that drugs, medical devices, and cosmetics are produced in a controlled environment and adhere to high-quality standards. GMP compliance is essential for both domestic and international trade.
- Quality Control: The manufacturing unit must have an established quality control system to test and ensure that each product batch meets the required specifications. This includes lab testing, stability studies, and adherence to safety protocols.
- Record-Keeping: Manufacturers must maintain detailed records of production processes, raw materials used, and finished products. These records must be readily available for inspection by regulatory authorities.
- Environmental and Safety Standards: Manufacturing facilities must adhere to environmental and safety regulations, including waste management and employee safety standards.
- Periodic Inspections and Audits: CDSCO may conduct periodic inspections and audits to ensure ongoing compliance with regulatory standards.
Challenges and Considerations
- Complex Regulatory Process: The process of obtaining a CDSCO manufacturing license can be complex and time-consuming. Manufacturers must be prepared to navigate regulatory requirements and complete all necessary paperwork accurately.
- High Costs: Setting up a manufacturing facility that meets the required standards for CDSCO approval can be expensive. This includes the costs of infrastructure, equipment, and personnel.
- Changes in Regulations: Regulatory requirements may change over time, which can necessitate adjustments in the manufacturing process or facility. Manufacturers must stay informed about updates in regulatory policies.
- Stringent Inspections: The inspections conducted by CDSCO are thorough, and any non-compliance can result in delays or rejection of the license application.
Conclusion
The CDSCO manufacturing license is a crucial document for any pharmaceutical or medical device manufacturer in India. It ensures that products meet the highest standards of safety, quality, and efficacy. Manufacturers must comply with a wide array of regulations and quality assurance standards to obtain and maintain this license. By doing so, they not only ensure the safety of their consumers but also gain access to the Indian market and international trade opportunities. It is important for manufacturers to thoroughly understand the regulatory requirements and adhere to them to avoid complications and penalties.